In an exciting partnership, FORCE is happy to announce five exclusive benefit screenings of the feature film Decoding Annie Parker. The film, starring Helen Hunt and Samantha Morton, tells the story of the discovery of BRCA1 and its influence on a family deeply affected by cancer. Screenings will take place in Chicago (July 25), Los Angeles (September 17), Washington DC (October 1), Philadelphia (October 2) and Palm Beach (October 12).
Click here to purchase tickets to a screening of Decoding Annie Parker
Read more!
Friday, June 28, 2013
Genetic Counselor, Ellen T. Matloff, Explains How Gene Patents Harmed Her Patients
The Supreme Court’s unanimous decision to overturn patents on human genes is a victory for genetic counselors, patients and advocacy groups alike. Ellen Matloff, Director of the Yale Cancer Genetic Counseling Program and plaintiff on this historical case, shares her perspective on the harmful effects such patents can have and how this ruling has opened the gateway toward increased opportunities for genetic testing.
Click here to read the original article posted by Breast Cancer Action Read more!
Click here to read the original article posted by Breast Cancer Action Read more!
Tuesday, June 25, 2013
WEBINAR THIS AFTERNOON: Supreme Court Decision to Overturn Human Gene Patents | Breast Cancer Action
Come ask all your questions about the Supreme Court's decision to overturn human gene patents. It's a huge win, but what does it mean for our health?
And if you're on Twitter, live-tweet with us during the webinar using #hgpwebinar.
Join us and a panel of experts to learn about the huge implications of this watershed decision for women’s health and beyond.
http://www.bcaction.org/
he
Supreme Court ruled in our favor to strike down Myriad Genetics’
patents on the human “breast cancer” genes, BRCA1 and BRCA2. This is a
tremendous win for women’s health – and for all our health!
We challenged Myriad's control of the BRCA genes because we couldn’t sit idly by while Myriad’s monopoly harmed women. Thank you for standing with us against corporate control of our health.
- See more at: http://www.bcaction.org/#sthash.KPQKelev.dpuf
We challenged Myriad's control of the BRCA genes because we couldn’t sit idly by while Myriad’s monopoly harmed women. Thank you for standing with us against corporate control of our health.
- See more at: http://www.bcaction.org/#sthash.KPQKelev.dpuf
 Read more!
Read more!
Wednesday, June 19, 2013
Monday, June 17, 2013
U.S. Supreme Court Liberates Breast Cancer Gene
Written by genetics law super expert Lori Andrews. Excerpt: "...paying Myriad nearly $4000 for each look at your breast cancer genes was like having to pay a car thief for the right to drive your own car."
read the full article here:
http://blogs.kentlaw.iit.edu/islat/2013/06/13/u-s-supreme-court-liberates-breast-cancer-gene/ Read more!
read the full article here:
http://blogs.kentlaw.iit.edu/islat/2013/06/13/u-s-supreme-court-liberates-breast-cancer-gene/ Read more!
Join the College of American Pathologists THIS WEDNESDAY, JUNE 19, for a one-hour audio conference on the Supreme Court decision from 3-4
Genomic medicine has the potential to be a cornerstone of medical testing, treatment, and clinical integration.
Without doubt, the Supreme Court’s unanimous decision invalidating
Myriad’s patents on BRCA1 and BRCA2 is already changing the testing
landscape for those genes. What other changes can we expect? How will
the decision impact pathology and our patients? What did it take to win
the case?
As a co-plaintiff in this landmark case, the College of American Pathologists (CAP) and all pathologists have cause to celebrate the Court's ruling. The CAP has assembled a panel of leading experts, including the researcher who discovered BRCA1 and BRCA2, and the ACLU attorney who argued for the plaintiffs and won. Listen as they, along with two leading molecular genetic pathologists, discuss the Supreme Court decision and the impact it will have on patient care.
https://www1.gotomeeting.com/register/414588793 Read more!
As a co-plaintiff in this landmark case, the College of American Pathologists (CAP) and all pathologists have cause to celebrate the Court's ruling. The CAP has assembled a panel of leading experts, including the researcher who discovered BRCA1 and BRCA2, and the ACLU attorney who argued for the plaintiffs and won. Listen as they, along with two leading molecular genetic pathologists, discuss the Supreme Court decision and the impact it will have on patient care.
https://www1.gotomeeting.com/register/414588793 Read more!
Thursday, June 13, 2013
A Day I Will Never Forget
Thursday, June 13 – a
day I will never forget. Today was my daughter's last day of nursery
school. I dropped her off, drove to a nearby coffee shop to get some
writing done, and then rejoined the class for parent sing-a-long and snack.
After the party my daughter and I ran through the rain to our car and I
then noticed that my abandoned cell phone was going crazy. Voicemails,
texts --- all saying the same thing: We won the Supreme Court BRCA Patent case
unanimously.
I immediately started
crying.
"Mommy, what's
wrong?"
"Nothing is wrong,
honey. Mommy is very happy."
"Why?"
"I won a fight
today that I've been fighting for a long time. This is going to help
Mommy's patients."
And then I thought of
all of my patients over the past 18 years and their children, and
grandchildren.
Generations that span
into the future --- all of whom will be helped by this ruling.
I cried harder as we
drove home through the rain, thinking of 15 years of fighting for a cause that
had all come to a beautiful end.
Ellen T. Matloff, MS
Research Scientist,
Department of Genetics
Director, Cancer Genetic
Counseling
Yale Cancer Center
Read more!
Professional Societies Applaud Supreme Courts Decision

|
Quest Diagnostics to Offer BRCA Testing
Quest Diagnostics Inc. (DGX), one of the largest laboratory diagnostics companies, plans to offer tests to identify the BRCA1 and BRCA2 genetic mutations thought to predict certain types of breast cancer after the Supreme Court ruled that genes can not be patented.
Quest's entry into the market could pressure sales for Myriad Genetics Inc. (MYGN), currently the only testing company to offer BRCA tests in the U.S. However, the Supreme Court unanimously ruled Thursday that human genes isolated from the body can't be patented, opening up the potential for competition to Myriad's BRCA tests.
"Based on our initial review of the Court's decision," said Wendy H. Bost, Quest's director of media relations, in an email, "we expect it will open opportunities for Quest Diagnostics to develop new testing
services, including in the area of hereditary breast cancer." As a result, the statement continued, "we now intend to validate and offer a BRCA1 and BRCA2 test service to physicians and patients later
this year."
Shares of Quest Diagnostics rose 1.4% to $62.88; meanwhile, Myriad shares fell 6.7% to $31.60. Earlier, Myriad's stock had risen as much as 13% because the Supreme Court also upheld Myriad's patents for cDNA, a synthetic version of the genes, but other methods for analyzing the genes are available to testing companies, analysts said.
http://online.wsj.com/article/BT-CO-20130613-710005.html
Read more!
Quest's entry into the market could pressure sales for Myriad Genetics Inc. (MYGN), currently the only testing company to offer BRCA tests in the U.S. However, the Supreme Court unanimously ruled Thursday that human genes isolated from the body can't be patented, opening up the potential for competition to Myriad's BRCA tests.
"Based on our initial review of the Court's decision," said Wendy H. Bost, Quest's director of media relations, in an email, "we expect it will open opportunities for Quest Diagnostics to develop new testing
services, including in the area of hereditary breast cancer." As a result, the statement continued, "we now intend to validate and offer a BRCA1 and BRCA2 test service to physicians and patients later
this year."
Shares of Quest Diagnostics rose 1.4% to $62.88; meanwhile, Myriad shares fell 6.7% to $31.60. Earlier, Myriad's stock had risen as much as 13% because the Supreme Court also upheld Myriad's patents for cDNA, a synthetic version of the genes, but other methods for analyzing the genes are available to testing companies, analysts said.
http://online.wsj.com/article/BT-CO-20130613-710005.html
Read more!
The Supreme Court unanimously ruled Thursday that human genes CANNOT be patented
Cancer counselor 'thrilled' with ruling on gene patents
By Carole Bass ’83, ’97MSL | 12:25pm June 13 2013
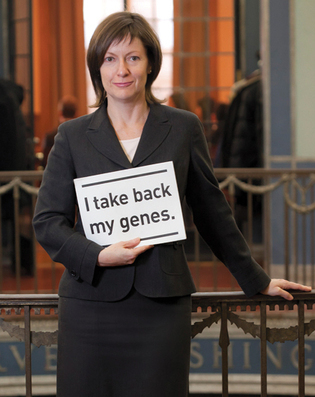
Yale Cancer Center's Ellen Matloff. Photo: Terry Dagradi
A US Supreme Court decision today means "cheaper, faster and better genetic testing" for Yale cancer patients, says Ellen Matloff, director of genetic counseling at the Yale Cancer Center and a plaintiff in the case.
In Association for Molecular Pathology v. Myriad Genetics, Matloff and others raised the question of whether human genes can be patented. The court's unanimous answer: no.
That means that Yale and other health care providers can begin offering their own genetic tests to compete with those sold by Myriad.
Immediately at issue were Myriad's patents on BRCA1 and BRCA2, the genes whose mutations (recently spotlighted by Angelina Jolie) predict high risk of breast and ovarian cancer. Utah-based Myriad claimed exclusive rights to test those genes and, according to Matloff, stopped Yale from offering a less expensive version.
"I'm thrilled," Matloff says by e-mail. "Our patients will have cheaper, faster and better genetic testing moving forward and research will open for BRCA carriers. Thrilled."
Matloff and other plaintiffs contended that genes are naturally occurring and therefore not patentable. Myriad responded that by isolating the DNA, it had created a new product to which it owned legal rights.
“Myriad did not create anything," Justice Clarence Thomas ’74JD concluded in the Supreme Court opinion. "To be sure, it found an important and useful gene, but separating that gene from its surrounding genetic material is not an act of invention."
The court did hold that cDNA, a synthetic version that omits certain portions of the original material, "is distinct from the DNA from which it was derived" and can be patented.
In a press release, Myriad claimed victory. "We believe the court appropriately upheld our claims on cDNA, and underscored the patent eligibility of our method claims, ensuring strong intellectual property protection for our BRACAnalysis test moving forward," says CEO Peter Meldrum. "More than 250,000 patients rely upon our BRACAnalysis test annually, and we remain focused on saving and improving peoples' lives and lowering overall healthcare costs." Read more!
By Carole Bass ’83, ’97MSL | 12:25pm June 13 2013
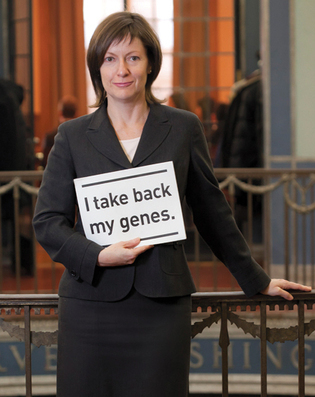
Yale Cancer Center's Ellen Matloff. Photo: Terry Dagradi
A US Supreme Court decision today means "cheaper, faster and better genetic testing" for Yale cancer patients, says Ellen Matloff, director of genetic counseling at the Yale Cancer Center and a plaintiff in the case.
In Association for Molecular Pathology v. Myriad Genetics, Matloff and others raised the question of whether human genes can be patented. The court's unanimous answer: no.
That means that Yale and other health care providers can begin offering their own genetic tests to compete with those sold by Myriad.
Immediately at issue were Myriad's patents on BRCA1 and BRCA2, the genes whose mutations (recently spotlighted by Angelina Jolie) predict high risk of breast and ovarian cancer. Utah-based Myriad claimed exclusive rights to test those genes and, according to Matloff, stopped Yale from offering a less expensive version.
"I'm thrilled," Matloff says by e-mail. "Our patients will have cheaper, faster and better genetic testing moving forward and research will open for BRCA carriers. Thrilled."
Matloff and other plaintiffs contended that genes are naturally occurring and therefore not patentable. Myriad responded that by isolating the DNA, it had created a new product to which it owned legal rights.
“Myriad did not create anything," Justice Clarence Thomas ’74JD concluded in the Supreme Court opinion. "To be sure, it found an important and useful gene, but separating that gene from its surrounding genetic material is not an act of invention."
The court did hold that cDNA, a synthetic version that omits certain portions of the original material, "is distinct from the DNA from which it was derived" and can be patented.
In a press release, Myriad claimed victory. "We believe the court appropriately upheld our claims on cDNA, and underscored the patent eligibility of our method claims, ensuring strong intellectual property protection for our BRACAnalysis test moving forward," says CEO Peter Meldrum. "More than 250,000 patients rely upon our BRACAnalysis test annually, and we remain focused on saving and improving peoples' lives and lowering overall healthcare costs." Read more!
Monday, June 3, 2013
Subscribe to:
Comments (Atom)



